Product
Product Center
 Diagnosis
Diagnosis





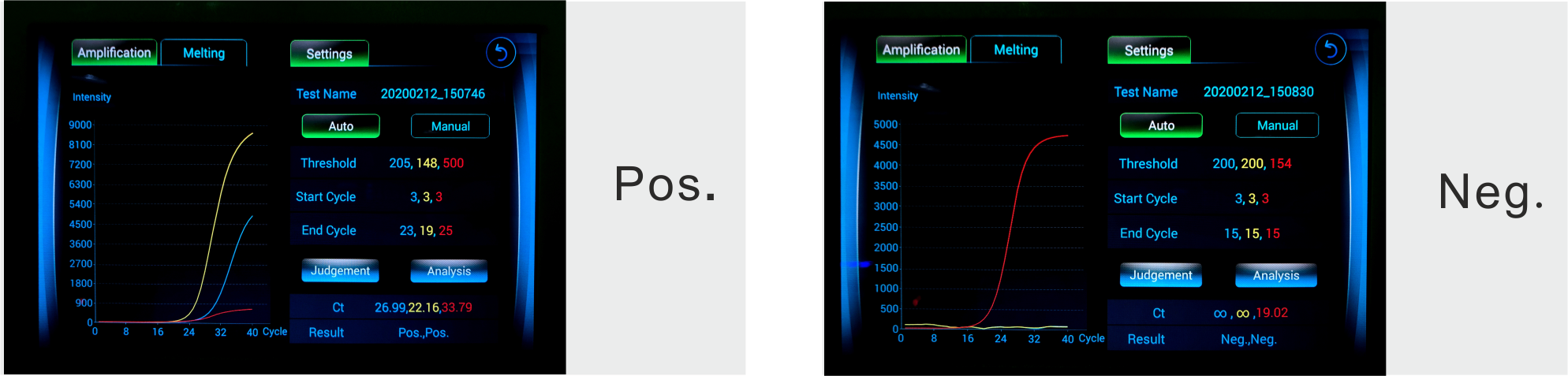
The kit is used to qualitatively detect the S gene and N gene of the new coronavirus from the suspected cases of pneumonitis, patients with suspected clustering cases, and other kinds of pharyngeal swabs and sputum samples from patients undergoing diagnosis or differential diagnosis of new coronavirus infection.
The samples could be the throat swab, sputum, alveolar lavage fluid or stool.
At present, the Automatic Nucleic Acids Detection System and supporting detection kits are used in CDCs and some clinical units in China.
There has more than 200 sets of the system installed in the hospitals and clinical units. And LifeReal supplies around 5000 tests per day.

Safe---The sealed kit to prevent the detection of pathogen leakage, reduce contact with infectious samples, and protect medical personnel
Simple---Manual sample operation in 2 minutes, training to the medical staff just in 10 minutes
Convenient---No PCR laboratory is required, no contamination
High sensitivity---Critical state multiphase microfluidic technology is used to effectively improve the efficiency of nucleic acid extraction, and the detection sensitivity is high up to 250 virus copies.
| Name | Storage | Package | Order No. | Note |
|
COVID-19 nucleic acid POCT detection kit (AIGS real-time fluorescent PCR method) |
2-8℃ |
24T |
C0011-E |
AIGS-LifeReady 1000 |
|
COVID-19 nucleic acid detection kit(Real-time fluorescent PCR method) |
-20℃ |
96T |
C0011-b |
Traditional realtime PCR machine |
 Back
Back