+86-571-87118973
FDA of Real-Time Fluorescent Quantitative PCR System is gotten !
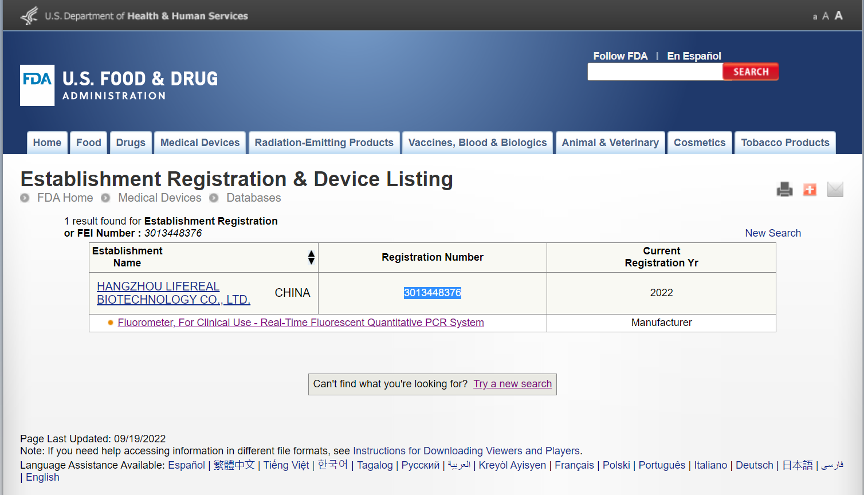
Registration number: 3013448376.
Remarkable news! Lifereal has got the FDA certificate for our new products, Real-Time Fluorescent Quantitative PCR System(QuantReady k9600), in September,2022.
QuantReady K9600 is a 96-well touchscreen quantitative fluorescence Real-time PCR system which has dual operating system.
Find us at the FDA website: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm